electron configuration for cr3+|2.6: Electron Configurations : Clark In order to write the Mg electron configuration we first need to know the . The Taiwan Dollar is currently stronger than the Japanese Yen, as 1 TWD is equal to 4.4754 JPY. Conversely, 1 JPY is worth 0.2234 TWD. Is the Taiwan Dollar up or down against the Japanese Yen? The Taiwan Dollar has decreased -2.57% year-to-date against the Japanese Yen.
PH0 · What is the electron configuration of Cr 3+?
PH1 · What is the electron configuration of Cr
PH2 · Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH3 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH4 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH5 · Electron Configuration For Chromium
PH6 · Electron Configuration For Chromium
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Electron Configuration Calculator
PH9 · Chromium
PH10 · 2.6: Electron Configurations
Pounds, dollars, pesos galore. Currency Converter is an exchange rate information and news app only and not a currency trading platform. The information shown there does not constitute financial advice. Conversion rates US Dollar / Philippine Peso; 1 USD: 56.45000 PHP: 5 USD: 282.25000 PHP: 10 USD: 564.50000 PHP: 20 USD: 1,129.00000 PHP:
electron configuration for cr3+*******The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .
Correct Electron Configuration for Copper (Cu) Half-filled and fully filled subshell .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .
In order to write the Mg electron configuration we first need to know the .

In writing the electron configuration for Potassium the first two electrons will go . To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons for the Cr atom. Mar 23, 2023 Chromium is known for its unique electron configuration, which deviates from the standard rules due to its half-filled and fully filled subshells. Let’s dive deeper into the details of Chromium’s electron configuration. Key Takeaways: Chromium’s electron configuration is . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.electron configuration for cr3+Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe temperature at which the liquid–gas phase change occurs.By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .
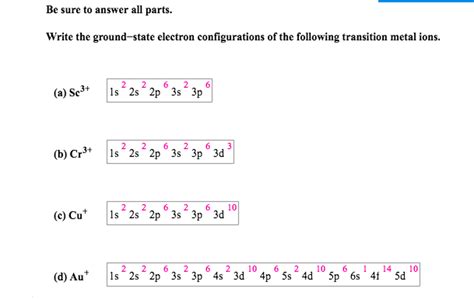
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .There is an apparent anomaly in the electron configuration for chromium. Cr is [Ar]3d 5 4s 1 and not [Ar]3d 4 4s 2. because an inner half–filled 3d sub–shell seem to be a little lower in energy, and marginally more stable. There is an apparent anomaly in the electron configuration for copper. Cu is [Ar]3d 10 4s 1 and not [Ar]3d 9 4s 2The electronic configuration of C r (24) atom is: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 which is half-filled d-orbital. C r 3 + has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [A r] 3 d 3.
The electronic configuration of Cr having atomic number of 24 is 1s22s22p63s23p64s13d5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3. Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .Choose the ground state electron configuration for Cr3+.Group of answer choices[Ar][Ar]4s23d1[Ar]4s13d2[Ar]4s23d6[Ar]3d3 Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.The Cr3+ ion has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 3d^3; These electron configurations for Chromium ions provide important insights into the distribution, orbital arrangement, and subshell filling of electrons, contributing to our understanding of the ion’s chemical properties.Write a complete electron configuration for an atom of titanium in the ground state. Write the ground-state electron configurations of the following ions: Li+, N-3, S-2, Al+3, Ba+2, Pb+2; Write an electron configuration for an atom of tin. Write complete electron configuration for element 'C'.
Question: 3) Choose the ground state electron configuration for Cr3+. A) [Ar]451302 B) [Ar] C) [Ar]452306 D) [Ar]3d3 E) [Ar]4s2301 . Show transcribed image text. There are 2 steps to solve this one. Step 1. Cr A 3 + It is chromium with 3 electrons removed from it. View the full answer. Step 2. Unlock. The ground state electron configuration for Cr3+ is [Ar]3d34s0. Explanation: The ground state electron configuration for Cr3+ is [Ar]3d34s0. Cr3+ has lost three electrons from its neutral atom, chromium (Cr) The electron configuration of chromium is [Ar]3d54s1, so when it loses three electrons, the 3d sublevel is filled and .electron configuration for cr3+ 2.6: Electron Configurations Question: Which of the following is the correct ground state electron configuration for Cr3+ ? [Ar]4 s2 [Ar]3 d3 [Ar]4s23d4 [Ar]3d2. . Answer :- In chemistry the electronic configuration is the distribution or arrangement of electrons in an atom. .View the full answer. Previous question Next .
Writing out the electron configuration tells us how the electrons in an atom or ion are arranged in their shells, subshells and orbitals; This can be done using the full electron configuration or the shorthand version. The full electron configuration describes the arrangement of all electrons from the 1s subshell up; The shorthand electron .
However, sometimes we also refer to the "degeneracy" of electron configurations. It is important to recognize that ground state electron configuration with \(M_s=m_s=+\frac{1}{2}\) is singly degenerate, and the same is true for \(M_s=m_s=-\frac{1}{2}\). Recognising degeneracy of electron configurations will be useful later in .2.6: Electron Configurations Electron configuration [Ar] 3d 5 4s 1 CAS number: 7440-47-3 ChemSpider ID: 22412: ChemSpider is a free chemical structure database . The Cr3+ ion is about 26% bigger than the Al3+ ion it replaces. So, when more chromium is added to aluminium oxide, the octahedral environment around the chromium becomes distorted and the two bands of .
Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the periodic table below; meaning n = 4 for the s-orbital.In addition, since we know that the energy level for the d orbital is "n-1", therefore .NOTE: Copper is an exception to the rules for writing electron configurations! Video: Cu, Cu +, and Cu 2+ Electron Configuration Notation In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital.
OKEBET Online Casino allows you to easily cash in /cash out via Gcash. We offering the most popular games including Slots, Live Casino, Sabong Baccarat, etc. OKEBET is the most turstworthy platform with the most members in Philippines. Parnertship with major game provider like JILI, Fa Chai, Evolution Gaming, AstroTech, CQ9, etc.!
electron configuration for cr3+|2.6: Electron Configurations